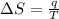Answer:
b. second law of thermodynamics
Step-by-step explanation:
Entropy (S) is a measure of the extent of disorder or randomness of a system. Greater the disorder, higher (more positive) will be the entropy.
According to the second law of thermodynamics the energy of an isolated system always increases and evolves towards thermal equilibrium. Since the universe is essentially an isolated system, it implies that the entropy of the universe is always increasing.
The change in entropy (ΔS) is given in terms of the ratio of thermal energy of the system (q) and temperature (T). SI unit is J/K