Alpha emission results in the daughter nuclide with atomic number reduced by 2 units and mass number reduced by 4 units.
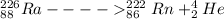
Beta emission results in the nuclide in which the atomic number is increased by one unit and the mass number remains unchanged.
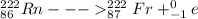
The resulting nuclide after the alpha and beta decay of Radium-226 will be Francium - 222