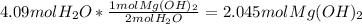Given volume of water produced in the reaction = 74.7 mL
Density of water = 0.987 g/mL
Calculating the mass of water =
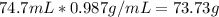
Moles of water produced =
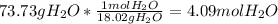
The balanced chemical equation between nitric acid and magnesium hydroxide is,

1 mol Magnesium hydroxide gives 2 mol water.
Moles of Magnesium hydroxide =