Answer: -
0.0506 M
Explanation:-
Let the volume of the solution be 1000 mL
Total mass of the solution = Density of the solution x Volume of the solution
= 1.10 g / mL x 1000 mL
= 1100 g.
Molality is the number of moles present per 1000 g of water.
Molality =0.0460 m
1000 g of the solution has 0.046 moles of NaBr.
1100 g of the solution has
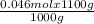
= 0.0506 mol
We started with 1000 mL solution.
We found it to contain 0.0506 mol
Molarity is defined as number of moles of NaBr per 1000mL of the solution.
Molarity of NaBr = 0.0506 M