Let us first find out the radioactive constant of Phosphorus 32.
Radioactive constant, λ =
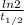
Here,
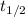 is half life of phosphorus 32 = 14.3 days
is half life of phosphorus 32 = 14.3 days
λ =
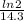
The amount of 4 mg of phosphorus remain after 71.5 days can be found using the formula,
m=m₀e⁻(λt)
=
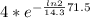
=
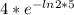
=
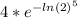
=
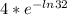
=
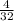
= 0.125 mg
The mass of 4 mg of phosphorus 32 remains after 71.5 days will be 0.125 mg.