Answer:
The internal pressure on the bottle of soda is
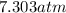
Step-by-step explanation:
We know that ''torr.'' is actually Torricelli a pressure unit. In order to find this pressure in ''atm'' (which is another pressure unit) we need to know the conversion factor.
The conversion factor is the following :
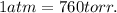
If we use the conversion factor to find the pressure in atm :
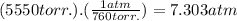
We found out that
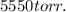 are equal to
are equal to
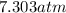
You can find the conversion factor on any units table.