Answer:- Solubility of KCl solution is 0.36 M.
Solution:- 2 kg of KCl dissolved into
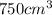 .
.
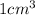 = 1 mL
= 1 mL
So,
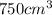 = 750 mL
= 750 mL
1000 mL = 1 L
So, 750 mL = 0.75 L
1 kg = 1000 g
So, 2 kg of KCl = 2000 g of KCl
Molar mass of KCl is 74.55 gram per mol.
So, moles of KCl =
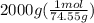
= 26.8 mol
Molar solubility of KCl =
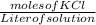
molar solubility of KCl =
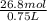
molar solubility of KCl = 0.36 M