Answer:
4.62kJ
Step-by-step explanation:
The heat is required for
Q1 = Heat required for conversion of solid to liquid
As there is no change in temperature so no heat will be absorbed in increasing the temperature.
Q1 = moles of water X heat of fusion of water
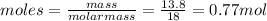
Q1= 0.7667 X6.03kJ/mol = 4.62kJ