So let's convert this amount of mL to grams:
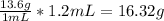
Then we need to convert to moles using the molar weight found on the periodic table for mercury (Hg):
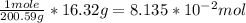
Then we need to convert moles to atoms using Avogadro's number:
![(6.022*10^(23)atoms)/(1mole) *[8.135*10^(-2)mol]=4.90*10^(22)atoms](https://img.qammunity.org/2019/formulas/chemistry/middle-school/e9khevnpc72qttzrnz661252ubt7f6sr5r.png)
So now we know that in 1.2 mL of liquid mercury, there are
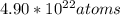 present.
present.