So first we must find how many moles this amount of atoms is. We know that Avogadro's number is the amount of atoms per mole:
![(1mole)/(6.022x10^(23) atoms) *[1.3*10^(21)atoms]=2.16*10^(-3)mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/qjg5o6bzdyc7jniq87jkqha64xoiszpohd.png)
So then we look to the periodic table to find the molar weight of sodium (Na).
Sodium's molar weight is 22.99g/mol. So let's solve for the amount of grams:
![(22.99g)/(1mol)*[2.16*10^(-3)mol]=4.97*10^(-2)g](https://img.qammunity.org/2019/formulas/chemistry/middle-school/4wa0dwmw13hgzg9gt2o2aa8izflb9j3d7s.png)
So now we know that the given amount of atoms weighs
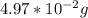 .
.