Answer : The correct answer for mass of material = 26.6 Kg
Given : Q = 4720 KJ
Change in Temperature ( ΔT ) = 65 K
Specific heat capacity of material ( c) = 2730
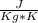
The relation between Q , ΔT , c and m are related by following formula :
Q = m* c*ΔT ,
where Q = Heat or energy absorbed or released
m = mass , c = specific heat ,ΔT = change in Temperature
Plugging value in heat formula :
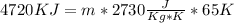
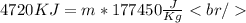
[[Converting
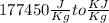
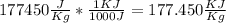 ]]
]]
Dividing both side by
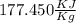
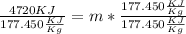
m = 26.6 Kg