The new pressure will be 8 kPa.
Explanation
Lets assume, the pressure of the gas is P and volume is V.
P is inversely proportional to V means :
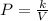 , where
, where
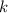 is a proportional constant.
is a proportional constant.
First, it is given that, Volume of hydrogen is 3.67 liter with a Pressure of 4 kPa
Now plugging V= 3.67 and P= 4 in the above equation:
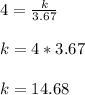
Now, we are moving all of the hydrogen to a 1.835 liter container. That means now the Volume is 1.835 liter
Plugging V= 1.835 and k= 14.68 into the original equation
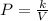 ,
,
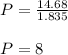
So, the new pressure will be 8 kPa.