Since 569324 mL = 569.324 L which is much more than 1.33 L that means it cannot be diluted to a volume of 1.33 L, so this must be 569.324 mL.
This question is solved by using the dilution equation which is :
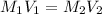
Where
 = Initial concentration
= Initial concentration
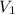 = Initial volume
= Initial volume
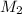 = Final concentration
= Final concentration
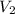 = Final volume
= Final volume
In our question , the given information is : V1 = 569.324 mL
M2 = 4.87 M and V2 = 1.33 L and we need to find
 .
.
When we use dilution equation we need to make sure that both the units of volume have same units.
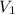 is in 'mL' and
is in 'mL' and
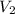 is in 'L' , so we will first convert
is in 'L' , so we will first convert
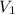 into 'L'
into 'L'
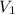 =
=
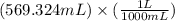
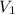 = 0.569324 L
= 0.569324 L
Now we will plug in the values of
 , in dilution equation and will calculate the value of
, in dilution equation and will calculate the value of
 .
.
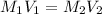
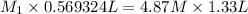
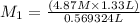
 (Initial concentration) = 11.38 M
(Initial concentration) = 11.38 M