Answer:
Nickel(II) phosphate is precipitated out.
Step-by-step explanation:
When ammonium phosphate and nickel(ii) nitrate are mixed together they precipitate out nickel(II) phosphate from the solution.
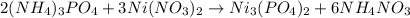
2 moles of ammonium phosphate and 3 moles of nickel(ii) nitrate gives 1 mol of nickel(II) phosphate and 6 moles of ammonium nitrate..