The reagent that can separate Fe³⁺ from Zn²⁺ is NaOH.
Explanation :
NaOH precipitates Fe³⁺ and Zn²⁺ ions in the form of their insoluble hydroxides.
The net ionic equations for these reactions are given below.
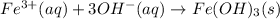
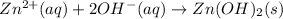
But when excess of NaOH is added, Zinc hydroxide forms a soluble complex ion known as "zincate" ion and gets dissolved in the solution . The reaction is given below.
![Zn^(2+) (aq)+ 4OH^(-)(aq)\rightarrow [Zn(OH)_(4)]^(2-)(aq)](https://img.qammunity.org/2019/formulas/chemistry/college/q1o27lzjll6sptjyq6adf60qg4ywolujlv.png)
Therefore in order to separate a mixture of Fe³⁺ and Zn²⁺, we have to add excess of NaOH and boil the mixture in water bath to ensure that the reaction goes to completion.
During this process, Fe³⁺ gets precipitated as Fe(OH)₃ whereas Zn²⁺ forms a soluble complex ion [Zn(OH)₄]²⁻ which remains in the solution.
When we filter the mixture, Fe³⁺ gets filtered off as a precipitate whereas Zn²⁺ remains in the solution. In this manner , the given ions can be separated.