Given information : Sample of
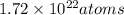
We need to find the moles of Chlorine in the given sample.
We can say that we need to find moles from the given atoms.
Relation between mole and atom is given by :
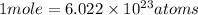
Where
 is Avogadro number.
is Avogadro number.
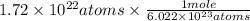
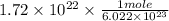
On solving the above equation , atoms(unit) gets cancelled out and we get 0.0286 mol.
In the given sample the moles of Chlorine (Cl) is 0.0286 mol , so option A is correct.