Answer:- Yes, strontum bromide and potassium sulfate gives a precipitate of strontium sulfate.
Explanations:- As per the solubility rules, all compounds of alkali metals are soluble.
Sulfate of most of the alkaline earth metals like Ca, Ba and Sr are insoluble.
A double displacement reaction takes place when strontium bromide and potassium sulfate are mixed and a precipitate of strontium sulfate is formed:
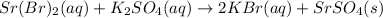
Note: (aq) stands for aqueous and (s) stands for solid and here it's precipitate.