The problem can be solved with the help of Michaelis Menten kinetics.
The equation can be written as
![V_(o)= (V_(max)[S])/(K_(m)+ [S])](https://img.qammunity.org/2019/formulas/chemistry/college/bo1nx5bnlw51iwelyhs321hwafv5o89tdf.png)
Where Vo = Initial velocity of the enzyme. This is also known as initial rate of the reaction.
Vmax = Maximum velocity
[S] = Substrate concentration
Km = Michaelis constant for the given enzyme.
When the substrate concentration is tripled, [S] becomes 3[S]
Let us plug this in Michaelis equation.
The new equation that we get is,
![V_(o)= (V_(max)* 3[S])/(K_(m)+ 3[S])](https://img.qammunity.org/2019/formulas/chemistry/college/cn76t7k2bwuv0snn81xela6natbarq1hht.png)
We have been given that Km = [S]
Let us write [S] in place of Km in our equation. We get,
![V_(o)= (V_(max)* 3[S])/([S]+ 3[S])](https://img.qammunity.org/2019/formulas/chemistry/college/kiefc2pb5yeqps4jxj0zm0zqgsdigdvjv8.png)
On adding the values on the denominator we get,
![V_(o)= (V_(max)* 3[S])/(4[S])](https://img.qammunity.org/2019/formulas/chemistry/college/ukh3z6lqkhdktv7fmv1cdfg4vpihbf4s5p.png)
We can cancel out [S] .
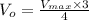
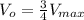
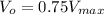
From the above equation, we can see that initial rate of the reaction (V₀) becomes 0.75 times Vmax
Therefore, when substrate concentration is tripled, the rate becomes 0.75 Vmax