Answer: -
Isopropyl alcohol = 308.95mL
Water = 526.05 mL
Explanation: -
Total volume = 835 mL
Percentage of Isopropyl alcohol = 37%
Volume of Isopropyl alcohol =
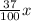 835 mL
835 mL
= 308.95 mL
Since volumes are additive,
Volume of water = Total volume - Volume of Isopropyl alcohol
= 835 - 308.95
= 526.05 mL
Thus, if Isopropyl alcohol is mixed with water to produce a 37.0% (v/v) alcohol solution. 526.05 mL water and 308.95 mL isopropyl alcohol are present in 835 ml of the solution (assuming that volumes are additive).