The given compound is
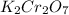
Each formula unit of
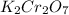 has 2 mol Potassium (K) atoms, 2 mol Chromium (Cr) atoms and 7 mol Oxygen (O) atoms.
has 2 mol Potassium (K) atoms, 2 mol Chromium (Cr) atoms and 7 mol Oxygen (O) atoms.
Atomic weight of potassium is 39.0983 amu
Atomic weight of Chromium is 51.9961 amu
Atomic weight of Oxygen is 15.9994 amu.
Therefore the formula weight of
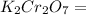
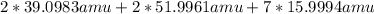
= 294.1846 amu