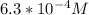pH is referred to as the scale to describe the hydronium ion concentration of a
solution. It is used to measure the acidity or basicity of a solution. pH of an acidic solution is less than 7, a neutral solution is 7, and a basic solution is greater than 7.
pH can be calculated using the formula:
![pH = - log [H_(3)O^(+)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/m4u0kjlewvkgqsoauyo3sd64e4u29bcahr.png)
Given the pH of solution is 3.2.
Finding out the concentration of hydronium ion from pH.
![pH = - log[H_(3)O^(+)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/f7fvot0cj6ixg97izz4vw5wr80w6mewoln.png)
![3.2 = - log [H_(3)O^(+)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/zxdx4k8a534t24khex977t63eaimpzuekd.png)
![[H_(3)O^(+)] = 10^(-3.2)](https://img.qammunity.org/2019/formulas/chemistry/high-school/v5aauqnvrjrswd13prgns9l5ea008xs7qf.png)
![[H_(3)O^(+)] = 6.3 * 10^(-4) M](https://img.qammunity.org/2019/formulas/chemistry/high-school/v7wp3rj2e03hk5bz5s0x7ohutq0ruiti6y.png)
Therefore, the hydronium ion concetration of the solution is