In electrolysis of water, water is decomposed into hydrogen and oxygen by passing a current.
Reaction at cathode :
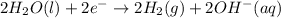
Reaction at anode :
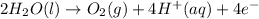
The net electrolysis reaction can be written as
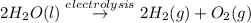
Step 1 : Find the coulombs of charge passed through the solution.
We will first find how many coulombs of charge passed through water for the given amount of time.
The formula that relates current in ampere and charge in coulombs is
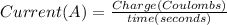
We have Current = 2 A
Time = 6 hrs 42 mins
Let us convert this to seconds.
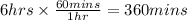
T = 360 mins + 42 mins = 402 mins
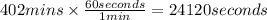
T = 24120 seconds
Let us plug in the values in the charge (Q) formula
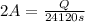
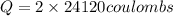
Q = 48240 C
Step 2 : Find moles of electrons transferred during the reaction.
According Faradays constant, 1 mol of electrons carry a charge of 96500 C.
We have 48240 C. Let us find how many moles of e- would carry this amount of charge.
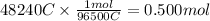
0.5 mol of electrons are transferred during the reaction.
Step 3 : Find moles of H2 using reaction at cathode.
From the reduction reaction occurring at cathode, we can see that 1 mol of H₂ gas is formed when 2 moles are electrons are accepted by water.
Therefore the mole ratio of e⁻ to H₂ is 2 : 1 . Let us use this as a conversion factor to find moles of H₂ from the calculated moles of electrons.
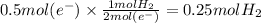
Moles of H₂ formed = 0.25 mol
Grams of H₂ =
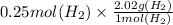
Grams of H₂ formed = 0.505 g
0.505 grams of H₂ are formed during the electrolysis reaction