Answer: D. It produces hydrogen ions in a solution.
Step-by-step explanation:
According to Arrhenius concept, a base is defined as a substance which donates hydroxide ions
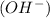 when dissolved in water and an acid is defined as a substance which donates hydrogen ions
when dissolved in water and an acid is defined as a substance which donates hydrogen ions
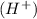 in water.
in water.
According to the Bronsted Lowry conjugate acid-base theory, an acid is defined as a substance which donates protons and a base is defined as a substance which accepts protons.
According to the Lewis concept, an acid is defined as a substance that accepts electron pairs and base is defined as a substance which donates electron pairs.
Hence, the correct statement is arrhenius acid produces hydrogen ions in solution.