Answer: -
If a gas at 250 K has a pressure of 0.00008 Pa and the pressure drops to 0.00002 Pa, 62.5 K is the new temperature
Explanation: -
Initial Pressure P₁ = 0.00008 Pa
Final Pressure P₂ = 0.00002 Pa
Initial Temperature T₁ = 250 K
Since the volume is not mentioned, it remains constant.
So initial Volume V₁ = Final volume V₂
Now using the combined gas equation
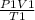 =
=
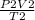
Final Temperature T₂ =
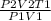
Plugging in the values,
T₂ =
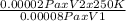
As V₂ = V₁, they cancel each other.
T₂ = 62.5 K