Answer: -
0.0583 g and 2.064 x 10²⁰ molecules of ammonia are released when a commercial refrigeration unit accidentally releases 7.68 x 10¹ mL of ammonia gas at STP
Explanation: -
Volume of ammonia gas released = 7.68 x 10¹ mL
= 76.8 mL
=
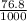
= 0.0768 L
We know that at STP standard temperature and pressure,
22.4 L of a gas has 6.02 x 10²³ molecules of ammonia.
0.0768 L of a gas is occupied by
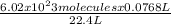
= 2.064 x 10²⁰ molecules of ammonia
Molar mass of NH₃ = 14 x 1 + 1 x 3 = 17 g.
At STP, we know
22. 4 L of ammonia has 17 g of ammonia.
0.0768 L of ammonia has
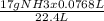
=0.0583 g of ammonia.
∴ 0.0583 g and 2.064 x 10²⁰ molecules of ammonia are released when a commercial refrigeration unit accidentally releases 7.68 x 10¹ mL of ammonia gas at STP