Answer:
0.9926 g/L is the density of gas.
Step-by-step explanation:
Molar mass of gas = M = 2.016 g/mol
Mass of gas = m
Density of the gas = d =
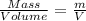 ...(1)
...(1)
Pressure of the gas = 4.77 atm
Temperature of the gas = T = 118 K
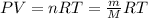
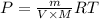
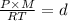 ..(From (1))
..(From (1))
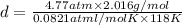
d = 0.9926 g/L
0.9926 g/L is the density of gas.