Answer:
See the answer below.
Step-by-step explanation:
The latent heat of fusion is defined as the amount of energy needed to make a phase change from solid to liquid. And it can be calculated by means of the following expression:
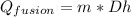
where:
Qfusion = Heat Energy [J]
m = mass = 0.65 [kg]
Dh = latent heat fusion = 330000[J/kg]
![Q_(fusion)=0.65*330000\\Q_(fusion)=214500[J]](https://img.qammunity.org/2022/formulas/physics/high-school/6wdn1psjchweycs3qdzio4wollsxv6mqff.png)
2)
We know that energy can be calculated from power, where power is defined as the relationship between energy over time.
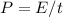
where:
P = power = 60 [w]
E = energy [J]
t = time = 5 [min] = 300 [s]
![E=P*t\\E=50*300\\E=15000[J]](https://img.qammunity.org/2022/formulas/physics/high-school/ksaoc5ts21xm9xg920d2f1pq0oz57nhlqc.png)
The mass change due to the process is equal to:
![m=282-274\\m=8[g]=0.008[kg]](https://img.qammunity.org/2022/formulas/physics/high-school/ziyytijj7jvqn8bkipkyyd4m1pp9s6yaij.png)
Now using the following equation for the latent heat of Fusion, we can calculate this value:
![Q=m*Dh\\Dh =Q/m\\Dh=15000/0.008\\Dh=1875000[J/kg]](https://img.qammunity.org/2022/formulas/physics/high-school/z7bhyxo5u55bwwt3hbendk3tod96q3d65s.png)