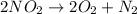Answer : The correct option is,
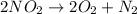
Explanation :
Decomposition reaction : It is a type of reaction in which a single larger compound decomposes to give two or more smaller molecules as a product.
The general representation of decomposition reaction is :
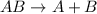
From the given options,
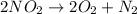 this reaction a decomposition reaction in which a single nitrogen dioxide decomposes to give oxygen gas and nitrogen gas as a product.
this reaction a decomposition reaction in which a single nitrogen dioxide decomposes to give oxygen gas and nitrogen gas as a product.
While the other reactions,
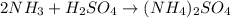 this reaction is a combination reaction in which the two or more molecules combine to form a larger single molecule as a product.
this reaction is a combination reaction in which the two or more molecules combine to form a larger single molecule as a product.
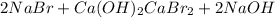 this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
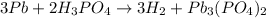 this is a single displacement reaction in which the more reactive metal displaces the least reaction metal from its compound.
this is a single displacement reaction in which the more reactive metal displaces the least reaction metal from its compound.
Hence, the decomposition reaction will be,