Answer: The mass of water released from the given amount of copper sulfate is 33.46 grams.
Step-by-step explanation:
We are given a compound having chemical formula
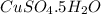
The molar mass of this compound =
![[63.55+32+(4* 16)+5(16+(2* 1))]=249.55g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/yk4368tzps346czgf2jxjo1w61lthrfzfd.png)
Mass of water molecule =
![[16+(2* 1)]=18g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/e5e685oqslur5j6pmy4854pw70qzdlj9us.png)
In 249.55 grams of the compound,
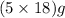 of water molecule is present.
of water molecule is present.
So, in 92.8 grams of the compound,
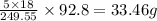 of water molecule.
of water molecule.
Hence, the mass of water released from the given amount of copper sulfate is 33.46 grams.