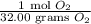Answer : The correct option is, (C)
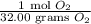
Explanation :
As we know that, the molar mass of
 is 32.00 gram per mole (32 g/mole).
is 32.00 gram per mole (32 g/mole).
To convert gram into mole, we use the conversion:
We are given the mass of
 is 106 grams.
is 106 grams.
Converting this into mole, we get:
As, 32 gram of
 present in 1 mole of
present in 1 mole of

And, 1 gram of
 present in
present in
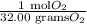
So, 106 gram of
 present in
present in
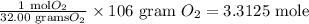
The equivalence factor use to convert gram to mole is,
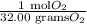
Hence, correct option is, (C)