Answer: -
O 2 limiting reagent
53.83 g CO2 theoretical Yield
97.9% percentage yield
Explanation: -
Mas of CH4 = 23.2 g
Molar mass of CH4 = 12 x 1 + 1 x 4 = 16g
Mass of O2 = 78.3 g
Molar mass of O 2 – 16 x 2 = 32 g
The balanced chemical equation for the reaction is
CH4 + 2 O2 = CO2 + 2 H2O
From the balanced equation we see that
2 O 2 reacts with 1 CH4
2 x 32 g of O 2 react with 16 g of CH4
78.3 g of O 2 react with
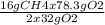
= 19.575 g pf CH4
Thus CH4 is in excess.
The limiting reagent is thus O 2.
Molar mass of CO2 = 12 x 1 + 16 x 2= 12 +32 = 44g
From the balanced equation we see
2O 2 gives 1 CO2
2 x 32 g of O 2 gives 44g of CO2
78.3 g of O 2 gives =
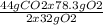
=53.83 g of CO2
Theoritical Yield = 53.83 g of CO2
Actual yield = 52.7 g
Percentage yield =
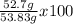
=97.9 %