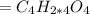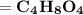Answer:
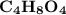
Step-by-step explanation:
From the given information:
Assume the molecular formula to be:
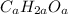
Then, the molecular mass will be = 12a + 2a + 16a = 30a (since the C = 12, H = 2 & O = 16) respectively.
Thus;
30a = 120
a = 120/30
a = 4
Thus, the molecular formula: