The reaction between zinc and silver nitrate is
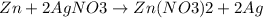
Moles of Zn = m /M = 599 /65 = 9.2 mol
Moles of AgNO3 = m /M = 1500 /169 = 8.87 mol
Silver nitrate is reacting completely to give silver
Thus, moles of Silver nitrate is equivalent to silver = 8.87
For mass of silver produced
m /M = 8.87 = m /107
m = 949.24 g
Hence, the given quantity of reactants are sufficient to produce 800 g of silver as the amount of silver produced is 949.24 g