Answer:
The particle momentum, p
Step-by-step explanation:
A particle's de Broglie's wavelength is an indication of the scale in length where the particle's wave-like properties are important. The symbol of de Broglie wavelength is λ or
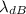 given as follows;
given as follows;
The de Broglie's wavelength formula is given as follows;
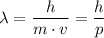
Where;
λ = The wavelength of the particle in meters
v = The velocity of the particle in meters/seconds
m = The mass of the particle in kilograms
p = The momentum of the particle
h = Planck's constant = 6.626 × 10⁻³⁴ J/Hz
Therefore, the alternative value that we must have to successfully determine the wavelength if the mass and velocity are unknown, is the momentum, p of the particle.