Answer:
0.67mole
Step-by-step explanation:
Given parameters:
Mass of potassium permanganate = 105.66g
Unknown:
Number of moles = ?
Solution:
To find the number of moles of the given compound;
Number of moles =
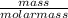
Molar mass of KMnO₄ = 39 + 55 + 4(16) = 158g/mol
Number of moles =
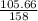 = 0.67mole
= 0.67mole