Answer:
4Fe + 3O₂ → 2Fe₂O₃
Step-by-step explanation:
The chemical reaction is given as:
Fe + O₂ → Fe₂O₃
To balance an equation implies that the number of atoms and moles must be conserved.
using the coefficients a,b and c, let use a mathematical approach to balance the expression;
aFe + bO₂ → cFe₂O₃
Conserving Fe: a = 2c
O: 2b = 3c
let c = 1, a = 2 , b =
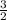
multiply through by 2 ;
a = 4, b = 3 and c = 2
4Fe + 3O₂ → 2Fe₂O₃