Answer: The correct answer is Option D.
Step-by-step explanation:
Noble gases are defined as the gases which have stable electronic configuration and is highly nonreactive. These belong to Group 18 of the periodic table.
For the given options:
The electronic configuration of hydrogen =
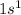
This element belongs to Group 1 and is known as alkali metal.
The electronic configuration of barium =
![[Xe]6s^2](https://img.qammunity.org/2019/formulas/chemistry/college/ve56mdldv6ax1tva19hr3a7i6js1o8cu4w.png)
This element belongs to Group 2 and is known as alkaline earth metal.
The electronic configuration of nitrogen =
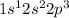
This element belongs to Group 15 and is known as p-block element.
The electronic configuration of krypton =
![[Ar]4s^23d^(10)4p^6](https://img.qammunity.org/2019/formulas/chemistry/college/l03khn2adu2gdj2z02tx4bweygqd2zxjeq.png)
This element belongs to Group 18 and is known as noble gas.
The electronic configuration of bromine =
![[Ar]4s^23d^(10)4p^5](https://img.qammunity.org/2019/formulas/chemistry/college/grmfu3kr1ezd08fugryz0tbzg63tdjc7qu.png)
This element belongs to Group 17 and is known as halogen.
Hence, from the above information, the correct answer is Option D.