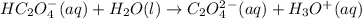Answer and Explanation:
The balanced chemical equations are as follows:
The chemical formula of oxalic is
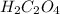
In the case when oxalic acts reacted with the water so here the oxalic acid eliminates one proton that leads to the development of mono acids
After that, the second step derives that when oxalic acid is in aqueous solution eliminates other proton so it represent the polyprotic acid
Now the chemical equations are as follows:
Elimination of one proton
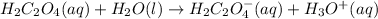
Now the elimination of other proton