Answer:- A.
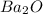 is not the correct formula.
is not the correct formula.
Explanations:- Charge for Ba is +2 and charge for O is -2, Here the charges are in equal and opposite so their ratio is 1:1 and hence the formula must be BaO and not
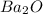 .
.
Rest of the formulas are correct as charge for Ca is +2 and charge for N is -3 . On criss cross we get the formula
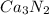 .
.
Charge for K is +1 and charge for Cl is -1 and so KCl is correct.
Charge for Li is +1 and that for S is -2. On criss cross we get the formula
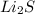 .
.