Answer:
50.8 g
Step-by-step explanation:
Equation of reaction.
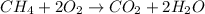
From the given information, the number of moles of methane = mass/ molar mass
= 15.4 g / 16.04 g/mol
= 0.960 mol
number of moles of oxygen gas = 90.3 g / 32 g/ mol
= 2.82 mol
Since 1 mol of methane requires 2 moles of oxygen
Then 0.960 mol of methane will require = 0.960 mol × 2 = 1.92 mol of oxygen gas
Thus, methane serves as a limiting reagent.
2.82 mol oxygen gas will result in 2.82 moles of water
So, the theoretical yield of water = moles × molar mass
= 2.82 mol × 18.01528 g/mol
= 50.8 g