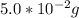look at yo periodic table to find the atomic mass
it says that there is 22.98g of sodium (Na) in 1 mol of sodium
1 mol is 6.022 * 10^23 atoms
one way we can do this is ratio and proportion
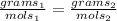
we are solving for
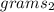
multiply both sides by
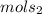
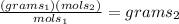
let's say
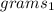 =22.98g and
=22.98g and
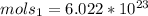 and
and
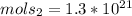
therefor
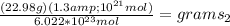
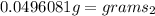
so it weighs about