Answer:
9.14moles
Step-by-step explanation:
Given parameters:
Number of atoms of butane = 5.5 x 10²⁴atoms
Unknown:
Number of moles = ?
Solution:
To solve this problem, we must understand that a mole of any substance contains the Avogadro's number of particles.
6.02 x 10²³ atoms makes up 1 mole of an atom
5.5 x 10²⁴ atoms will contain =
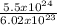 = 9.14moles
= 9.14moles