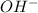The radical in this question is OH.
The reason this will be a radical, is that the definition of a radical is the presence of an unpaired electron. This causes the radical to be unstable, desperately wanting to do something with the free electron that it has.
Oxygen has a charge of 2-, and Hydrogen has a charge of +1. When you pair them, you end up with a net charge of 1-, which is the presence of that unpaired electron. It will usually be written as: