Answer:- Atomic number for sulfur is 16 and it's electron configuration is
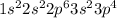 . Here, there are total for electrons in 3p and the set of quantum numbers for these 4 electrons would be as..
. Here, there are total for electrons in 3p and the set of quantum numbers for these 4 electrons would be as..
For the first electron of 3p-
n = 3, l = 1, ml = -1 and ms = +(1/2)
for the second electron of 3p-
n = 3, l = 1, ml = 0 and ms = +(1/2)
for the third electron of 3p-
n = 3, l = 1, ml = +1 and ms = +(1/2)
and for the fourth electron of 3p-
n = 3, l = 1, ml = -1 and ms = -(1/2)