Concentration (m) signify 'molality'
Concentration (M) signify 'Molarity'
Formula for molality (m) =
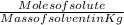
In the question denisty of solute is not given so we can calculate concentration (M) that is 'Molarity'
Molarity (M) =
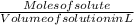
Moles of solute CH3OH = Given grams / Molar mass of CH3OH
Given grams of CH3OH = 12.9 g and molar mass = 32.0 g/mol
Moles of solute CH3OH =
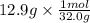
Moles of solute CH3OH = 0.403 mol
Volume of solution = 230 mL , we need to convert 230 mL to 'L'
Volume =
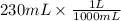
Volume = 0.23 L
Molarity (M) =
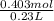
Concentration (M) = 1.75 mol / L or 1.75 M