Answer:- Theoretically 7.20 moles of
 are formed.
are formed.
Solution:- The balanced equation for the reaction of Sulfur with nitric acid is---
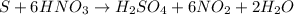
From balanced equation, 1 mol of sulfur reacts with 6 moles of nitric acid. So, 1.20 moles of sulfur would react with 6 x 1.20 = 7.20 moles of nitric acid.
9.90 moles of nitric acid are available. It means nitric acid is present in excess and sulfur is limited. So, the theoretical yield is calculated by 1.20 moles of sulfur as...
1.20 mol x (6 mol nitrogen dioxide/1mol S) = 7.20 mol
