Answer:
The first step would be to write the balanced chemical reaction
Step-by-step explanation:
Given:
Mass of Mg = 28.0 g
The first step is to write down the balanced chemical reaction depicting the stoichiometry
2Mg + O2 → 2MgO
Therefore, 2 moles of Mg produces 2 moles of MgO
i.e. the ratio of Mg:MgO = 1:1
Atomic mass of Mg = 24 g/mol
Moles of Mg reacted =
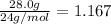
This is equal to the moles of MgO produced = 1.167
Molar mass of MgO = 24 + 16 = 40 g/mol
Mass of MgO produced = 1.167 moles * 40 g/mol = 46.7 g