Here by ideal gas equation we have
PV = nRT
so here if
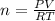
So we can say pressure , volume and temperature ratio is same then number of moles must be same
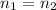
for
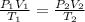
So number of moles is directly proportional to product of volume and pressure of the gas and inversely proportional to temperature of the gas