Answer: pOH of the solution will be 10.1
Step-by-step explanation:
pOH is defined as the negative logarithm of hydroxide ion concentration present in a solution. The equation used to represent pOH of the solution follows:
![pOH=-\log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/high-school/ur2f3m6zoirj5p05ac4nknmpiip97f0mi9.png)
We are given:
pH of the solution = 3.9
To calculate the pOH os the solution, we use the following equation:
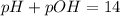
Putting values in above equation, we get:
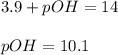
Hence, pOH of the solution will be 10.1