Hello!
The half-life is the time of half-disintegration, it is the time in which half of the atoms of an isotope disintegrate.
We have the following data:
mo (initial mass) = 43 g
m (final mass after time T) = ? (in g)
x (number of periods elapsed) = ?
P (Half-life) = 20 minutes
T (Elapsed time for sample reduction) = 80 minutes
Let's find the number of periods elapsed (x), let us see:
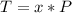
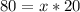
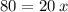
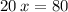
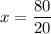
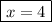
Now, let's find the final mass (m) of this isotope after the elapsed time, let's see:
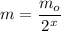
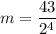
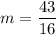

I Hope this helps, greetings ... DexteR! =)